21321.In which of the following ionization processes the bond energy increases and the magnetic behaviour changes from paramagnetic to diamagnetic?
N2 → N+
2
2
O2 → O+
2
2
C2 → C+
2
2
NO → NO+
21322.In allene (C3H4), the type(s) of hybridisation of the carbon atoms is/are
sp and sp3
sp and sp2
Only sp2
sp2 and sp3
21324.The number of electrons in the valence shell of the central atom of a molecule is 8. The molecule is
BeH2
SCl2
SF6
BCl3
21325.The sp3d2 hybridization of central atom of a molecule would lead to
square planar geometry
Tetrahedral geometry
Trigonal bipyramidal geometry
Octahedral geometry
21326.The number of types of bonds between two carbon atoms in calcium carbide is
Two sigma, two pi
One sigma, two pi
One sigma, one pi
Two sigma, one pi
21327.Which of the following has maximum number of lone pairs associated with Xe?
XeF2
XeO3
XeF4
XeF6
21328.Peroxide ion ______ .
a) is diamagnetic.
b) has five completely filled antibonding molecular orbitals.
c) is isoelectronic with neon.
d) has bond order one.
Which one of these is correct?
a) is diamagnetic.
b) has five completely filled antibonding molecular orbitals.
c) is isoelectronic with neon.
d) has bond order one.
Which one of these is correct?
a), b) and d)
d) and c)
a) and d)
a), b) and c)
21329.Which of the following is a favorable factor for cation formation?
Low ionisation potential
High electron affinity
High electronegativity
Small atomic size
21331.During the formation of a chemical bond
energy decreases
energy increases
energy of the system does not change
electron-electron repulsion becomes more than the nucleus-electron attraction
21332.Which of the following species contains three bond pairs and one lone pair around the central atom?
NH–
2
2
PCl3
H2O
BF3
21333.The correct order of decreasing H-C-H angle in the following molecules is
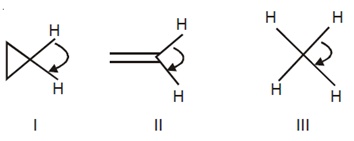

I > II > III
II > I > III
III > II > I
I > III > II
21334.NiCl2{P(C2H5)2(C6H5)}2 exhibits temperature dependent magnetic behaviour (paramagnetic/diamagnetic). The coordination geometries of Ni2+ in the paramagnetic and diamagnetic states are respectively
tetrahedral and tetrahedral
square planar and square planar
tetrahedral and square planar
square planar and tetrahedral
21336.Which one of the following pairs is isostructural (i.e. having the same shape and hybridization)?
[NF3 and BF3]
[BF–
4 and NH+
4]
4 and NH+
4]
[BCl3 and BrCl3]
[NH3 and NO–
3]
3]
21337.The state of hybridization of the central atom and the number of lone pairs over the central atom in POCl3 are
sp, 0
sp2, 0
sp3, 0
dsp2, 1
21338.When O2 is converted into O+
2
2
both paramagnetic character and bond order increase
bond order decreases
paramagnetic character increases
paramagnetic character decreases and the bond order increases
21339.Which of the following statement is correct?
The number of electrons present in the valence shell of S in SF6 is 12.
The rates of ionic reactions are very slow.
According to VSEPR theory, SnCl2 is the linear molecule.
The correct order of ability to form ionic compounds among Na+, Mg2+ and Al3+ is Al3+ > Mg2+ > Na+
21340.The states of hybridization of boron and oxygen atoms in boric acid (H3BO3) are respectively:
sp2 and sp2
sp2 and sp3
sp3 and sp2
sp3 and sp3
