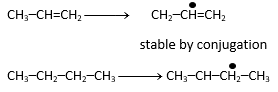
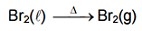|
Which one of the following compounds shows the presence of intramolecular hydrogen bond?
|
Answer
|
|
The molar conductivity of a 0.5 mol / dm3 solution of AgNO3 with electrolytic conductivity of 5.76 × 10-3 S cm-1 at 298 K is
|
Answer
|
|
The decomposition of phosphine (PH3) on tungsten at low pressure is a first-order reaction. It is because the
|
Answer
|
|
The coagulation values in millimoles per litre of the electrolytes used for the coagulation of As2S3 are given below:
I. (NaCl) = 52, II. (BaCl2) = 0.69 III. (MgSO4) = 0.22
The correct order of their coagulating power is
|
Answer
|
|
During the electrolysis of molten sodium chloride, the time required to produce 0.10 mol of chlorine gas using a current of 3 amperes is
|
Answer
|
|
How many electrons can fit in the orbital for which n = 3 and l = 1 ?
|
Answer
|
|
For a sample of perfect gas when its pressure is changed isothermally from pi to pf, the entropy change is given by
|
Answer
|
|
The vant Hoff factor (i) for a dilute aqueous solution of the strong electrolyte barium hydroxide is
|
Answer
|
|
The percentage of pyridine (C5H5N) that forms pyridinium ion (C5H5N+H) in a 0.10 M aqueous pyridine solution (Kb for C5H5N = 1.7 × 10–9) is
|
Answer
|
|
In calcium fluoride, having the fluorite structure, the coordination numbers for calcium ion (Ca2+) and fluoride ion (F–) are
|
Answer
|