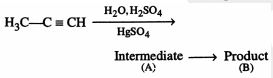
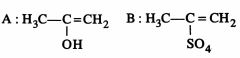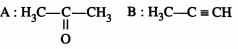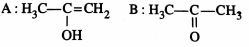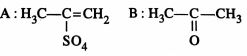46849.The root mean square velocity of an ideal gas at constant pressure varies with density (d) as
$d^2$
d
$\sqrt{d}$
$\frac{1}{\sqrt{d}}$
46851.In van der Waals equation of state for a non-ideal gas, the term that accounts for inter molecular forces is
(V—b)
$(RT)^{-1}$
$(P+\dfrac{a}{V^2})$
RT
46852.The reaction of white phosphorus with aqueous NaOH gives phosphine alongwith another phosphorus containing compound. The reaction type, the oxidation states of phosphorus in phosphine and the other product respectively are:
redox reaction, -3 and -5
disproportionation reaction, -3 and +5
redox reaction, 3 and +5
disproportionation reaction, -3 and +3
46854.The given graph represent the variations of z (compressibility factor (Z)= $ \dfrac{pV}{nRT}$ ) versus p, for three real gas A, B and C. Identify the only incorrect statement.
For the gas A, a = 0 and its dependence on p is linear at all pressure
For the gas B, b = 0 and its dependence on p is linear at all pressure
For the gas C, which is typical real gas for which neither a nor b = 0. By knowing the minima and the point of intersection, with Z = 1, a and b can be calculated
At high pressure, the slope is positive for all real gases
46855.In the dichromate dianion
4 Cr—O bonds are equivalent
6 Cr—O bonds are equivalent
all Cr-O bonds are equivalent
all Cr—O bonds are non-equivalent
46857.The packing efficiency of two-dimensional square unit cell shown below is
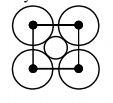

39.27%
68.02%
74.05%
78.54%
46858.Which of the following has longest C–O bond length? (free in C–O bond length in CO is 1.128 A.)
$[Co(CO)_4]^{-}$
$[Fe(CO)_4]^{2-}$
$[Mn(CO)_6]^{+}$
$Ni(CO)_4$
46860.Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because
zinc is lighter than iron
zinc is has lower melting point than iron
zinc has lower negative electrode potential than iron
zinc has higher negative electrode potential than iron
46861.For a sample of perfect gas when its pressure is changed isothermally from p; to pi, the entropy change is given by
$\triangle S = nRIn(\dfrac{pf}{p_i})$
$\triangle S = nRIn(\dfrac{p_i}{pf})$
$\triangle S = nRTIn(\dfrac{pf}{p_i})$
$\triangle S = RTIn(\dfrac{p_i}{pf})$
46863.In the following radioactive decay, $_{92}X^{232} \rightarrow _{89}Y^{220}$, how many $\\alpha$ and $\\beta$-particles are ejected from X to Y?
3$\alpha$ and 2$\beta$
5$\alpha$ and 3$\beta$
3$\alpha$ and 3$\beta$
5$\alpha$ and 5$\beta$
46864.A 0.0020 m aqueous solution of an ionic compound $Co[(NH_3)_5(NO_2)]Cl$ freeze at - 0.00732°C. Number of moles of ions which 1 mole of ionic compound produces on being dissolved in water will be (Ky = - 1.86°C/m)
2
3
4
1
46866.A compound contains atoms of three elements A, B and C, If the oxidation number of A is +2, B is +5 and that of C is -2, the possible formula of the compound is
$A_2(BC_3)_2$
$A3(BC_4)_2$
$A_3(B_4C)_2$
$AB_C2$
46867.The d-electron configuration of $Cr^{2+}, Mn^{2+} Fe^{2+} and Co^{2+} are d^4, d^5,d^6 and d^7$ drespectively. Which one of the following will exhibit minimum paramagnetic behaviour? (At. no. Cr = 24, Mn = 25, Fe = 26, CO = 27)
$[Fe(H_2O)_6]^{2+}$
$[Co(H_2O)_6]^{2+}$
$[Cr(H_2O_6)]^{2+}$
$[Mn(H_2O)]_6]^{2+}+$