A gas mixture consists of 2 moles of O2 and 4 moles of Ar at temperature T. Neglecting all
vibrational modes, the total internal energy of the system is
|
A body of mass 6 kg is acted upon by a force which causes a displacement in it given |
Answer |
|
Two rods of same length and area of cross-section and Young s moduli Y, and Y2 are joined |
Answer |
|
If a person can throw a stone to maximum height of h metre vertically, then the |
Answer |
|
A thin copper wire of length l increases in length by 1%, when heated from 0°C to 100°C. If |
Answer |
|
3 mole of hydrogen is mixed with 1 mole of neon. |
Answer |
|
3. Two conducting rings P and Q of radii r and 2r moving uniformly in opposite directions with |
Answer |
|
In an electromagnetic wave, the magnetic field is given by |
Answer |
|
A cubical vessel of height 1 m is full of water. What is the amount of work done in |
Answer |
|
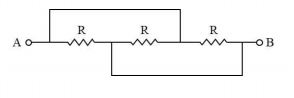
The resistance across A and B in the figure below will be |
Answer |
|
Using the Gibbs energy changes, AG° = + 63.3 kJ for the following reaction, |
Answer |