The pair of species with the same bond order is
|
Consider the following compounds: |
Answer |
|
The root mean square velocity of an ideal gas at constant pressure varies with density (d) as |
Answer |
|
Which of the following compound is a peroxide? |
Answer |
|
In van der Waals equation of state for a non-ideal gas, the term that accounts for inter molecular forces is |
Answer |
|
The reaction of white phosphorus with aqueous NaOH gives phosphine alongwith another phosphorus containing compound. The reaction type, the oxidation states of phosphorus in phosphine and the other product respectively are: |
Answer |
|
The substance used as a smoke screen in warfare is |
Answer |
|
The given graph represent the variations of z (compressibility factor (Z)= $ \dfrac{pV}{nRT}$ ) versus p, for three real gas A, B and C. Identify the only incorrect statement. |
Answer |
|
In the dichromate dianion |
Answer |
|
The species that do not contain peroxide ions, is |
Answer |
|
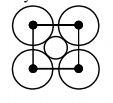
The packing efficiency of two-dimensional square unit cell shown below is |
Answer |