The reaction of white phosphorus with aqueous NaOH gives phosphine alongwith another phosphorus containing compound. The reaction type, the oxidation states of phosphorus in phosphine and the other product respectively are:
|
The substance used as a smoke screen in warfare is |
Answer |
|
The given graph represent the variations of z (compressibility factor (Z)= $ \dfrac{pV}{nRT}$ ) versus p, for three real gas A, B and C. Identify the only incorrect statement. |
Answer |
|
In the dichromate dianion |
Answer |
|
The species that do not contain peroxide ions, is |
Answer |
|
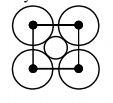
The packing efficiency of two-dimensional square unit cell shown below is |
Answer |
|
Which of the following has longest C–O bond length? (free in C–O bond length in CO is 1.128 A.) |
Answer |
|
The number of radial nodes in 3s and 2p respectively are |
Answer |
|
Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because |
Answer |
|
For a sample of perfect gas when its pressure is changed isothermally from p; to pi, the entropy change is given by |
Answer |
|
The number of geometrical isomers of the complex $[Co(NO_2)_3(NH_3)_3]$ is |
Answer |