The given graph represent the variations of z (compressibility factor (Z)= $ \dfrac{pV}{nRT}$ ) versus p, for three real gas A, B and C. Identify the only incorrect statement.
|
In the dichromate dianion |
Answer |
|
The species that do not contain peroxide ions, is |
Answer |
|
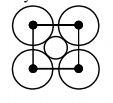
The packing efficiency of two-dimensional square unit cell shown below is |
Answer |
|
Which of the following has longest C–O bond length? (free in C–O bond length in CO is 1.128 A.) |
Answer |
|
The number of radial nodes in 3s and 2p respectively are |
Answer |
|
Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because |
Answer |
|
For a sample of perfect gas when its pressure is changed isothermally from p; to pi, the entropy change is given by |
Answer |
|
The number of geometrical isomers of the complex $[Co(NO_2)_3(NH_3)_3]$ is |
Answer |
|
In the following radioactive decay, $_{92}X^{232} \rightarrow _{89}Y^{220}$, how many $\\alpha$ and $\\beta$-particles are ejected from X to Y? |
Answer |
|
A 0.0020 m aqueous solution of an ionic compound $Co[(NH_3)_5(NO_2)]Cl$ freeze at - 0.00732°C. Number of moles of ions which 1 mole of ionic compound produces on being dissolved in water will be (Ky = - 1.86°C/m) |
Answer |