In the following radioactive decay, $_{92}X^{232} \rightarrow _{89}Y^{220}$, how many $\\alpha$ and $\\beta$-particles are ejected from X to Y?
|
A 0.0020 m aqueous solution of an ionic compound $Co[(NH_3)_5(NO_2)]Cl$ freeze at - 0.00732°C. Number of moles of ions which 1 mole of ionic compound produces on being dissolved in water will be (Ky = - 1.86°C/m) |
Answer |
|
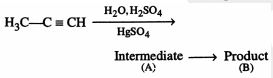
Predict the correct intermediate and product in the following reaction: |
Answer |
|
A compound contains atoms of three elements A, B and C, If the oxidation number of A is +2, B is +5 and that of C is -2, the possible formula of the compound is |
Answer |
|
The d-electron configuration of $Cr^{2+}, Mn^{2+} Fe^{2+} and Co^{2+} are d^4, d^5,d^6 and d^7$ drespectively. Which one of the following will exhibit minimum paramagnetic behaviour? (At. no. Cr = 24, Mn = 25, Fe = 26, CO = 27) |
Answer |
|
Which one is most reactive towards electrophilic reagent? |
Answer |
|
Taxonomically a species is ………. |
Answer |
|
Two plants can be conclusively said to belong to the same species if they ……. |
Answer |
|
Find the correct statements for the following |
Answer |
|
Where endangered animal species are kept for reproduction? |
Answer |
|
Unicellular eukaryotic microorganisms comprise ________ . |
Answer |