A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half. Then
|
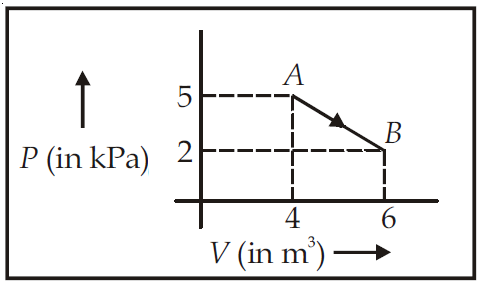
One mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in the figure, |
Answer |
|
The coefficient of performance of a refrigerator is 5. If the temperature inside freezer is –20°C, the temperature of the surroundings to which it rejects heat is |
Answer |
|
A piece of ice falls from a height h so that it melts completely. Only one–quarter of the heat produced is absorbed by the ice and all energy of ice gets converted into heat during its fall. The value of h is |
Answer |
|
An ideal gas heat engine operates in a Carnot cycle between 227ºC and 127ºC. It absorbs 6 kcal at the higher temperature. The amount of heat (in kcal) converted into work is equal to |
Answer |
|
Which statement is incorrect? |
Answer |
|
A refrigerator works between 4°C and 30°C. It is required to remove 600 calories of heat every second in order to keep the temperature of the refrigerated space constant. The power required is [Take 1 cal = 4.2 joules) |
Answer |
|
A Carnot engine, whose efficiency is 40%, takes in heat from a source maintained at a temperature of 500K. It is desired to have an engine of efficiency 60%, then the intake temperature for the same exhaust (sink) temperature must be : |
Answer |
|
An ideal gas is compressed to half its initial volume by means of several processes. Which of the process results in the maximum work done on the gas? |
Answer |
|
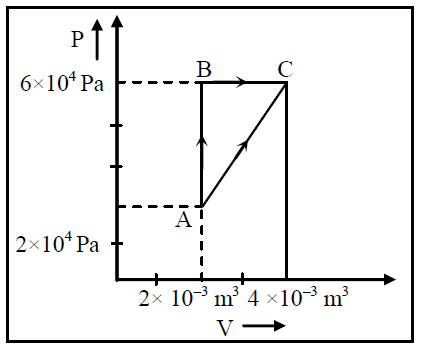
Figure below shows two paths that may be taken by a gas to go from a state A to a state C. In process AB, 400 J of heat is added to the system and in process BC, 100 J of heat is added to the system. The heat absorbed by the system in the process AC will be |
Answer |
|
A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half. Then |
Answer |