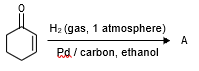|
Consider the reaction
CH3CH2CH2Br + NaCN → CH3CH2CH2CN + NaBr
This reaction will be the fastest in
|
Answer
|
|
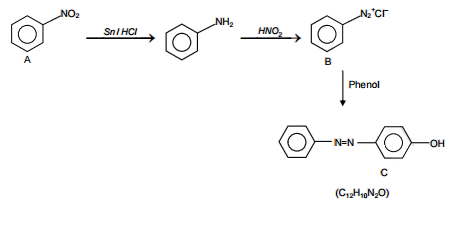
The correct structure of the product A formed in the reaction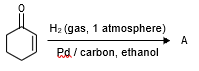
is
|
Answer
|
|
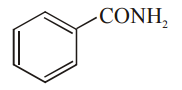
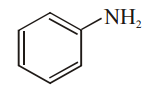
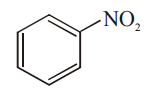
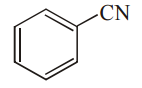
Which among the given molecules can exhibit tautomerism?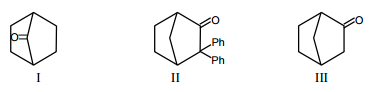
|
Answer
|
|
The correct order of strengths of the carboxylic acids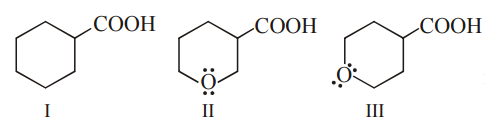
is
|
Answer
|
|
The compound that will react most readily with gaseous bromine has the formula:
|
Answer
|
|
Which one of the following compounds shows the presence of intramolecular hydrogen bond?
|
Answer
|
|
The molar conductivity of a 0.5 mol / dm3 solution of AgNO3 with electrolytic conductivity of 5.76 × 10-3 S cm-1 at 298 K is
|
Answer
|
|
The decomposition of phosphine (PH3) on tungsten at low pressure is a first-order reaction. It is because the
|
Answer
|
|
The coagulation values in millimoles per litre of the electrolytes used for the coagulation of As2S3 are given below:
I. (NaCl) = 52, II. (BaCl2) = 0.69 III. (MgSO4) = 0.22
The correct order of their coagulating power is
|
Answer
|
|
During the electrolysis of molten sodium chloride, the time required to produce 0.10 mol of chlorine gas using a current of 3 amperes is
|
Answer
|